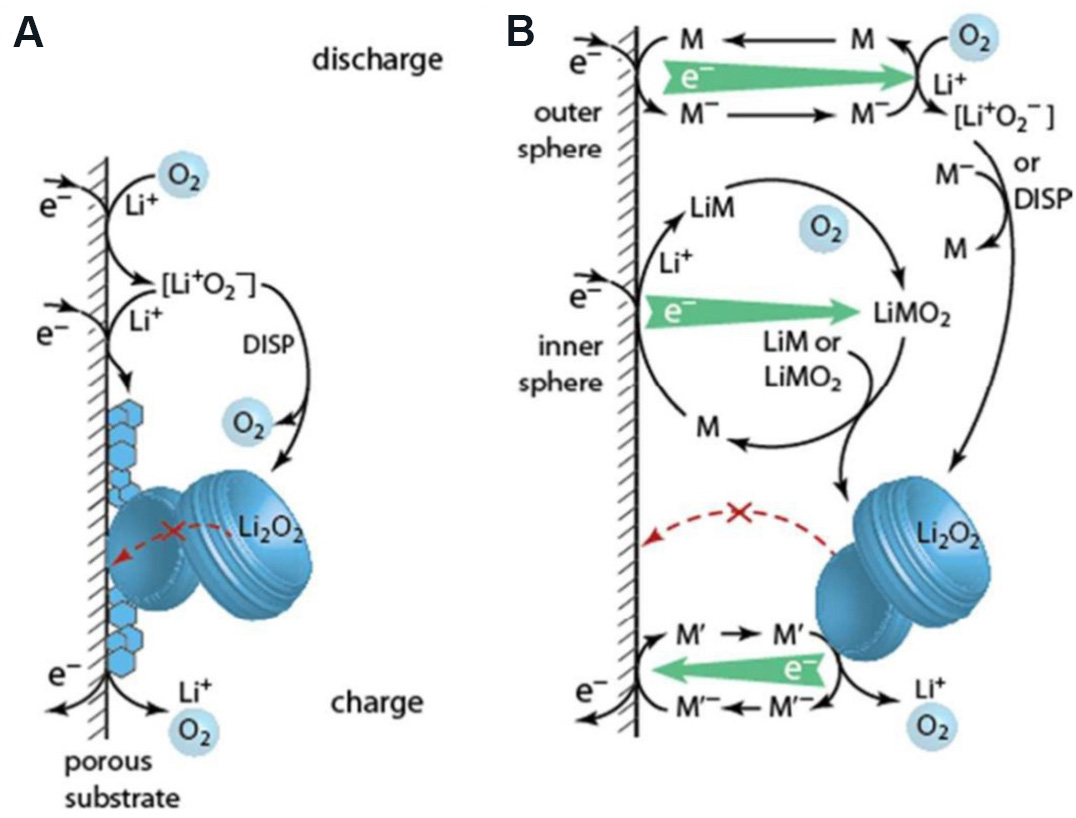
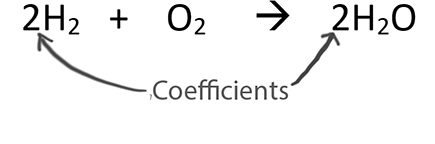Porous Materials Applied in Nonaqueous Li–O2 Batteries: Status and Perspectives - Wang - 2020 - Advanced Materials - Wiley Online Library

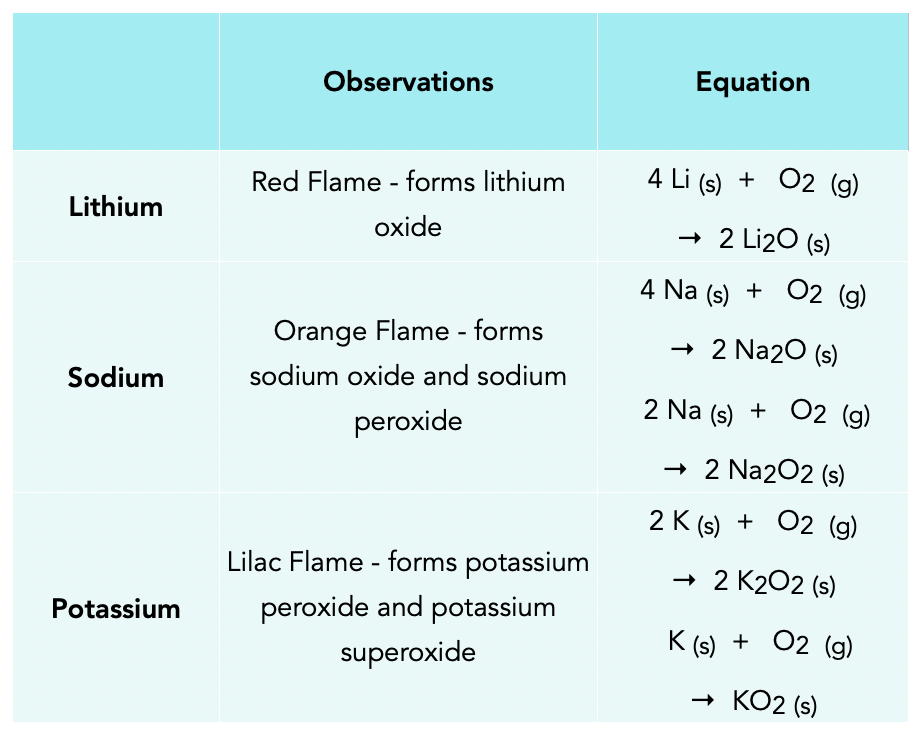
WARM UP 4 Li + O2 2 Li2O If you have 804 g of Li available for the reaction, calculate the amount of O2 you will need to pump into the
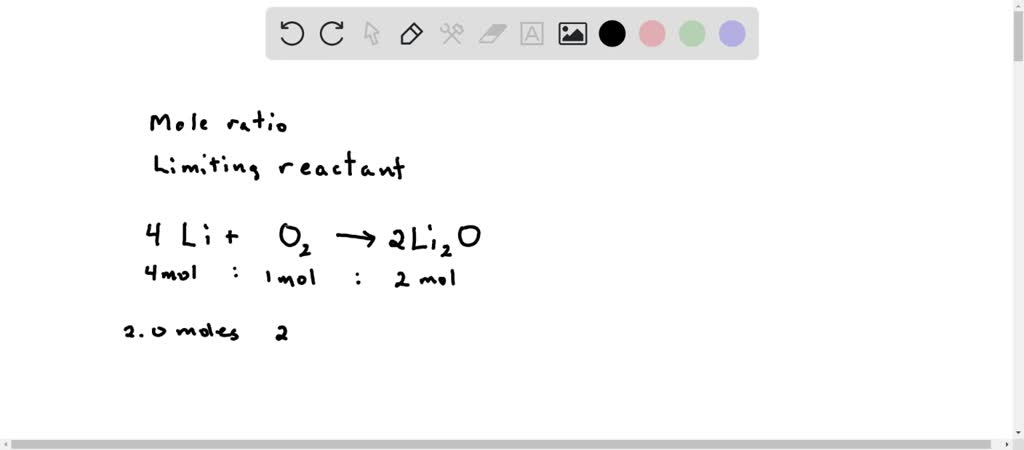
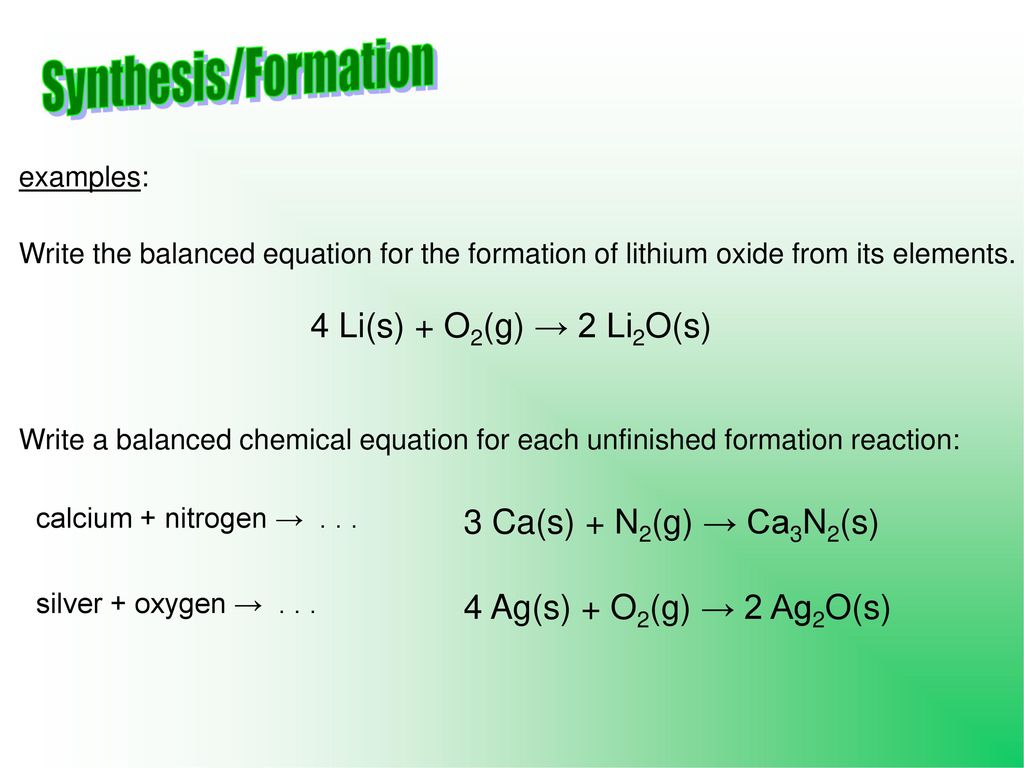
SOLVED: Lithium metal reacts with oxygen gas to form lithium oxide, according to the following unbalanced reaction: Li + O2 ? Li2O If 2.0 moles of Li react with 2.0 mol of
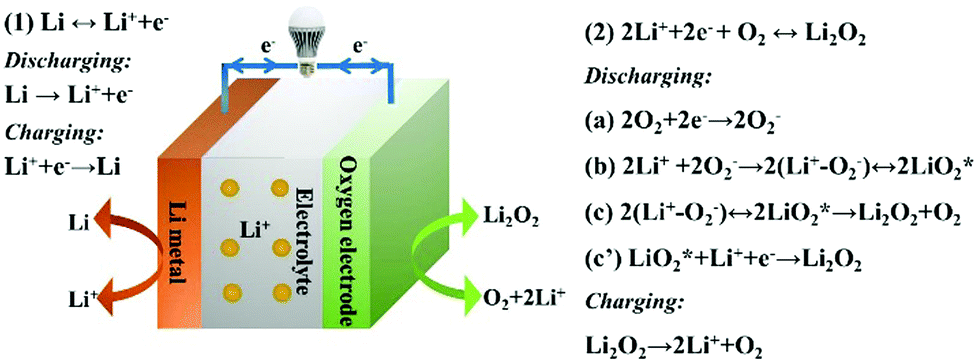
Functional and stability orientation synthesis of materials and structures in aprotic Li–O 2 batteries - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C8CS00009C

Lithium Peroxide Growth in Li–O2 Batteries via Chemical Disproportionation and Electrochemical Mechanisms: A Potential-Dependent Ab Initio Study with Implicit Solvation | The Journal of Physical Chemistry C

Porous Materials Applied in Nonaqueous Li–O2 Batteries: Status and Perspectives - Wang - 2020 - Advanced Materials - Wiley Online Library

Coupling solid and soluble catalysts toward stable Li anode for high-performance Li–O2 batteries - ScienceDirect

Density Functional Investigation of the Thermodynamic Stability of Lithium Oxide Bulk Crystalline Structures as a Function of Oxygen Pressure | The Journal of Physical Chemistry C

Lithium–Oxygen Battery Exploiting Highly Concentrated Glyme-Based Electrolytes | ACS Applied Energy Materials
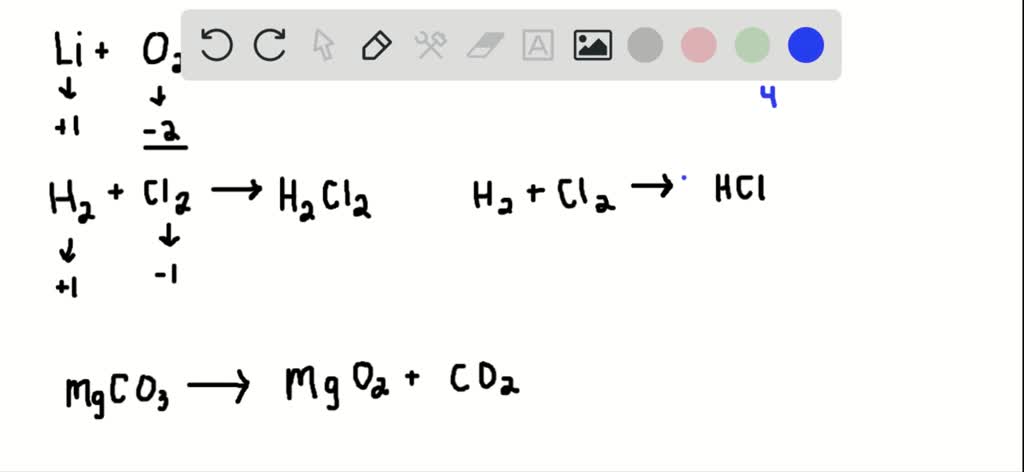
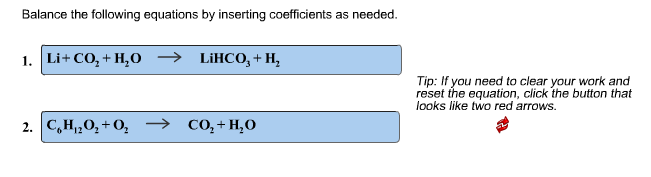
SOLVED: Identify and correct each error in the following equations, and then balance each equation. a. Li+O2⟶LiO2 b. H2+Cl2⟶H2Cl2 c. MgCO3⟶MgO2+CO2 d. NaI+Cl2⟶NaCl+I

Complete and balance the following equations :(a) Na + O2 → (b) Na2O + H2O → (c) Fe(s) + H2O(g) red heat (d) Cu(NO3)2 (aq) + Zn(s) →
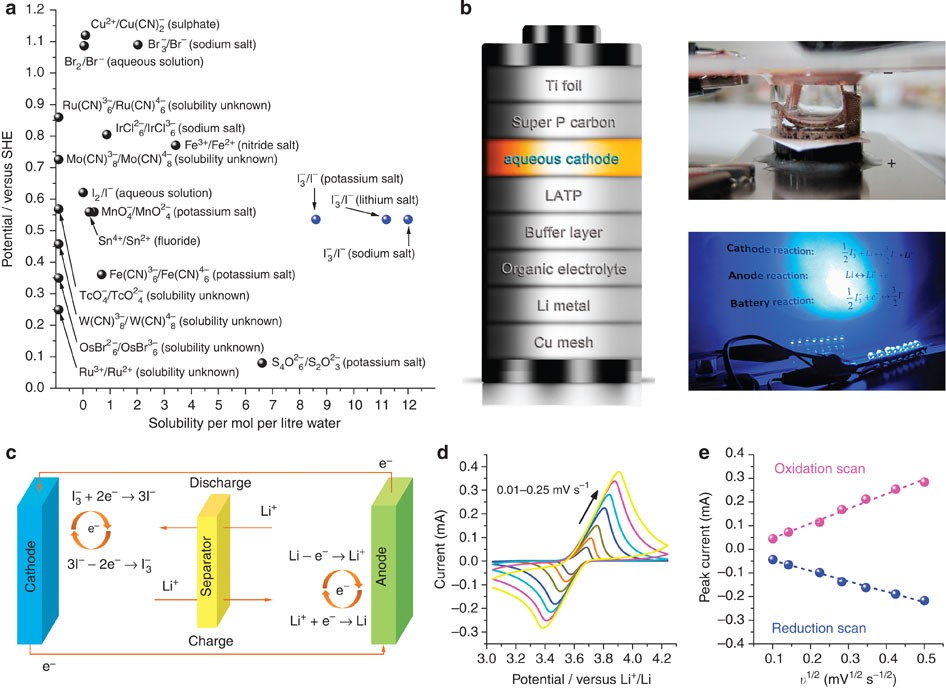
High-performance rechargeable lithium-iodine batteries using triiodide/iodide redox couples in an aqueous cathode | Nature Communications
![2KClO3 MnO2 2KCl + 3O2 Calculate the mass of KClO3 required to produced 6.72 litre of O2 at S.T.P. [K = 39, Cl = 35.5, O = 16]. 2KClO3 MnO2 2KCl + 3O2 Calculate the mass of KClO3 required to produced 6.72 litre of O2 at S.T.P. [K = 39, Cl = 35.5, O = 16].](https://dwes9vv9u0550.cloudfront.net/images/905096/dc069731-0770-457a-935a-fac1566c37d5.jpg)