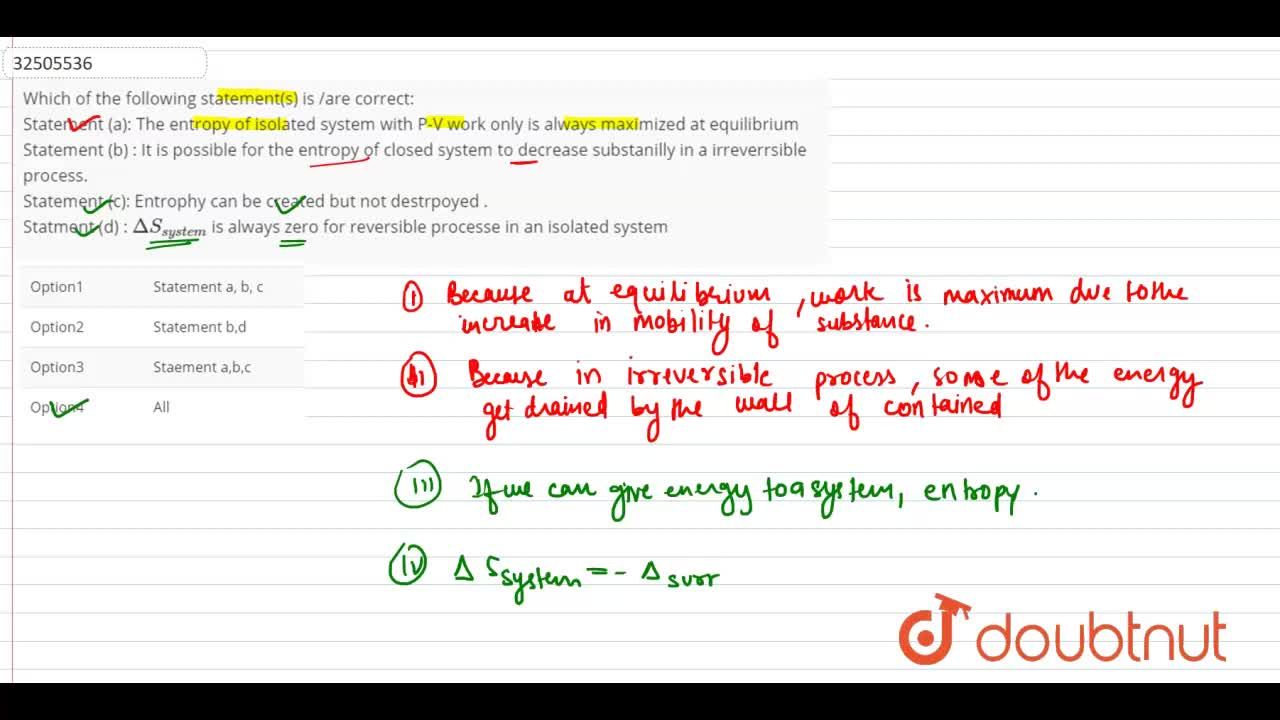
Student ideas regarding entropy and the second law of thermodynamics in an introductory physics course: American Journal of Physics: Vol 77, No 10

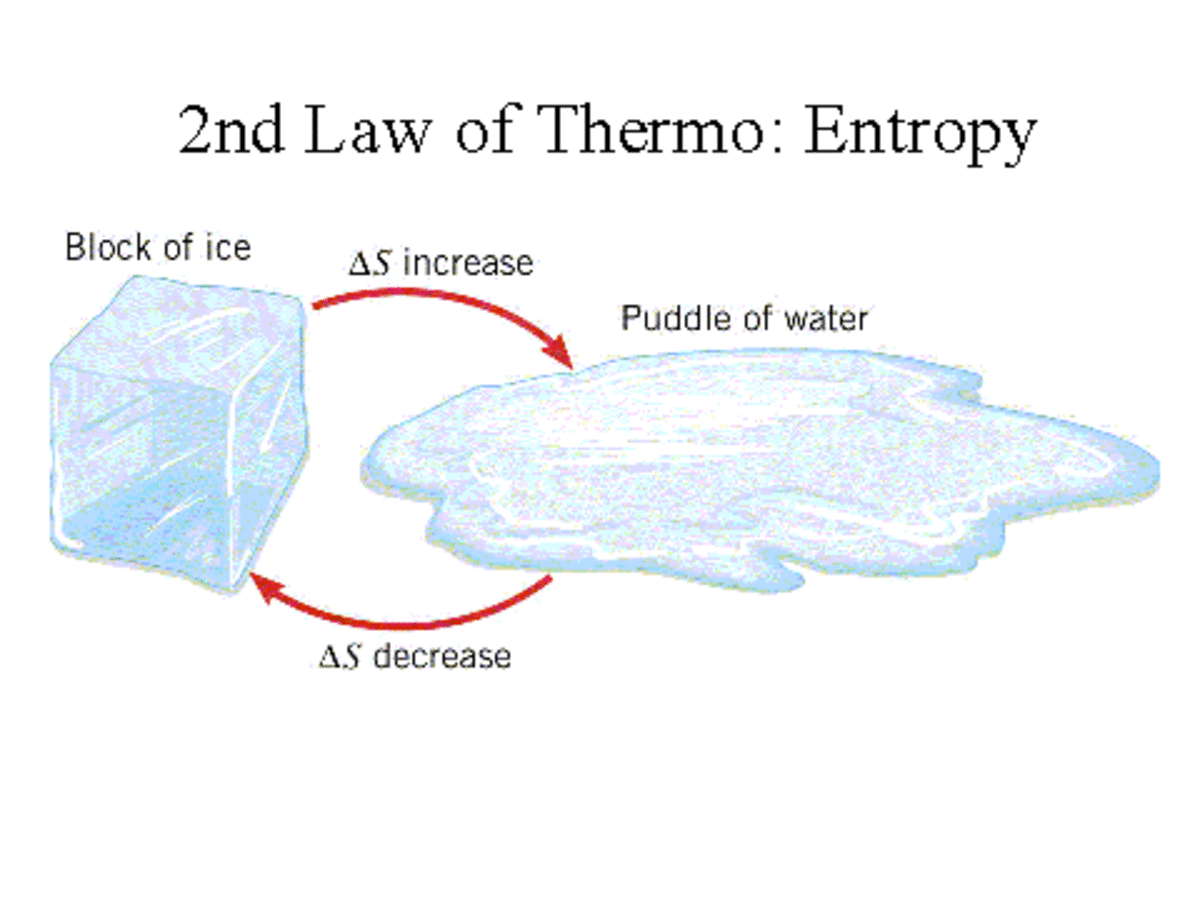
thermodynamics - Does entropy increase with a decrease or with an increase in a system's temperature? - Physics Stack Exchange

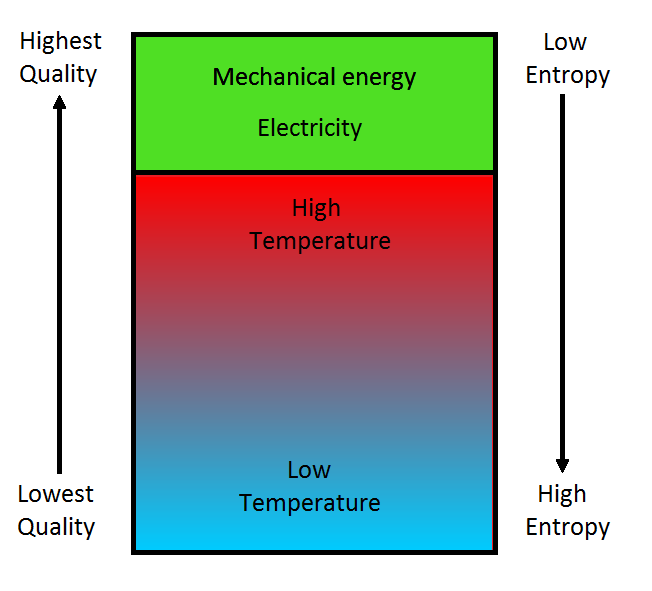
Chapter 15 The Laws of Thermodynamics. Thermodynamics n The study of the processes in which energy is transferred as heat and as work heat--transfer of. - ppt download
Student ideas regarding entropy and the second law of thermodynamics in an introductory physics course

Lecture 24: Entropy of an isolated system increases during irreversible process; Clausius inequality - YouTube

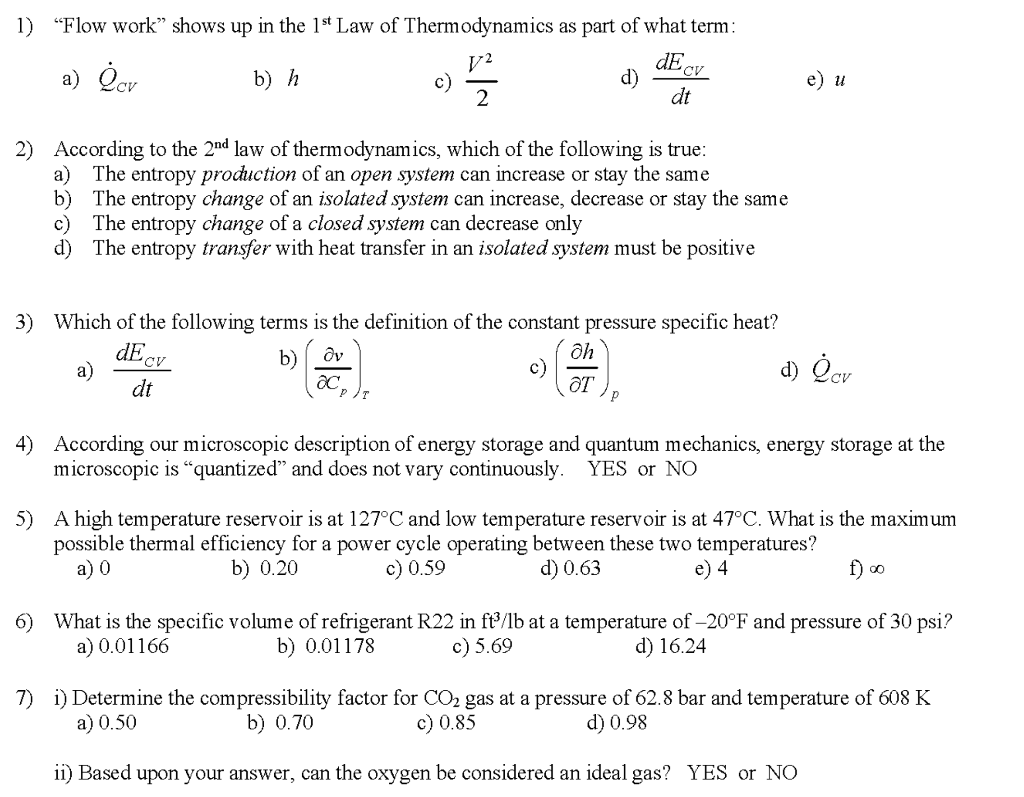
1 The Laws of Thermodynamics in Review 1.The internal energy* of an isolated system is constant. There are only two ways to change internal energy – heat. - ppt download
Entropy in an isolated system can only increase. Is this an empirical law, or is it a property that comes from the definition that uses W the number of possible states [S =